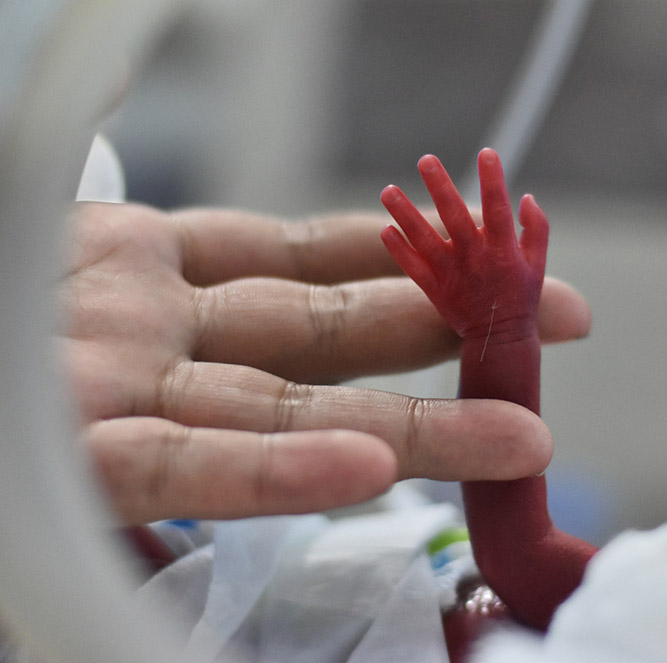
When it comes to preterm births, the World Health Organization estimates that 15 million babies are born before the completion of 37 weeks gestation. Preterm birth complications are the leading cause of death among children under five years of age, with approximately 1 million deaths in 2015. Strikingly, three-quarters of these deaths could be prevented with current, cost-effective interventions.
In an interview recorded at the Pediatric Academic Societies (PAS) meeting in Washington, DC, in May 2023, we spoke with Dr. Brett Manley on the research he is doing to add to those interventions. Dr. Manley is a Consultant Neonatologist at The Royal Women’s Hospital in Melbourne, Australia, and an Associate Professor in the Department of Obstetrics and Gynaecology at The University of Melbourne. The research he’s doing right now as the co-investigator of the PLUSS trial seeks to determine whether a particular two-drug protocol can lessen the effects or even prevent bronchopulmonary dysplasia (BPD), a common and serious complication in extremely preterm babies.
Carol Vassar, producer
Guest: Brett J. Manley, MBBS, FRACP, PhD, Consultant Neonatologist, The Royal Women’s Hospital, Melbourne, Australia
Subscribe, review or let your voice be heard at NemoursWellBeyond.org.
Episode Transcript
Carol Vassar, podcast host/producer:
Welcome to Well Beyond Medicine, the Nemours Children’s Health Podcast. Each week we’ll explore anything and everything related to the 80% of child health impacts that occur outside the doctor’s office. I’m your host, Carol Vassar. And now that you are here let’s go.
MUSIC: Well Beyond Medicine
Carol Vassar, podcast host/producer:
When it comes to preterm births, the World Health Organization estimates that 15 million babies are born before the completion of 37 weeks gestation. Preterm birth complications are the leading cause of death among children under five years of age, about 1 million deaths in 2015. Strikingly three-quarters of these deaths could be prevented with current cost-effective interventions. In a wide-ranging interview recorded at the meeting of the Pediatric Academic Societies, PAS, in Washington, D.C. in May 2023, I spoke with Dr. Brett Manley on the research he’s doing to add to those interventions. Dr. Manley is a consultant neonatologist at the Royal Women’s Hospital in Melbourne, Australia, and an associate professor in the Department of Obstetrics and Gynecology at the University of Melbourne. The research he’s doing right now is as the co-investigator of a study looking at whether a particular two-drug protocol can lessen the effects and even prevent Bronchopulmonary dysplasia or BPD, a common and serious complication in extremely preterm babies. Dr. Manley provides details on his research and the struggles of finding families willing to partake in research generally. But first, let’s get a clinical definition of BPD and how treating it has vexed neonatologists for decades. Here’s Dr. Brett Manley.
Dr. Brett Manley, University of Melbourne:
Yeah. BPD, Bronchopulmonary dysplasia, and even easier if we refer to it as chronic lung disease of prematurity. So this is a condition that affects at least half, and probably more than that, of all extremely preterm babies. So these are the really little babies born more than three months early, or we would say, before 28 weeks gestation. So these little babies have a really difficult journey starting their life and through their stay in the Neonatal Intensive Care Unit. And that generally involves quite a lot of respiratory support and oxygen therapy to help with their immature lungs. And lots of those babies need support for a long time and oxygen for a long time. And if they reach 36 weeks and are still needing significant support, we diagnose them with this condition, Bronchopulmonary dysplasia. That condition in itself or that diagnosis may not be critically important so much to families, but what is is we know that BPD is associated with worse long-term outcomes as well, and that stands to reason. The sicker babies that need more help with their breathing need more oxygen, need more respiratory support, do have more trouble in the long term, and that’s both developmental and general health, but also respiratory and breathing problems.
And it’s sort of the bane of the neonatologist’s existence and for the families and babies as well that this is such a common condition that we’re having a lot of trouble preventing or improving. And there are very few therapies that seem to be able to do that in our armament. One therapy that’s useful is corticosteroids. These are drugs that are used for many things in medicine to reduce inflammation. And similarly, they can be used in extremely preterm babies to reduce inflammation in the lungs, get them off mechanical ventilators and improve their outcomes. But like a lot of medications and therapies used in neonatology, we’ve learned the hard way that there’s no easy answer. Giving systemic steroids into the bloodstream or into the stomach are associated with worse long-term outcomes themselves, and so we are really careful in how we use those and only in the sickest babies with BPD or chronic lung disease.
So we’re desperately looking for new ways to prevent or improve the severity of BPD. And one way that’s been described is actually giving it directly into the lungs. So the idea there is that instead of giving into the bloodstream where it can go all over the body and affect other organs, most importantly the brain, and have adverse effects, if you could give it straight into the lungs, that would mean it works in the organ that you’re trying to target in the lungs and hopefully have less side effects. We didn’t come up with this idea. There’s a famous doctor from Taiwan called Dr. Wei who’s here at the conference today who’s previously presented a small randomized trial looking at the concept of combining steroid medication called Budesonide with another commonly used drug called Surfactant that we give to pretty much all of our extremely preterm babies and squirting that down into the lungs.
And he’s showed some preliminary findings that that’s very, very effective at reducing BPD. And in fact, just yesterday at this very conference, he presented a second trial very similar to the first showing again a very strong effect on BPD. So we’ve seen this as have a lot of clinicians around the world, and I guess to be honest, we felt maybe that huge effect that he has seen in those two smaller trials needs to be replicated in a larger trial in different babies using different techniques. And that’s where we’ve designed the PLUSS trial. So this is a huge randomized trial of over a thousand extremely preterm infants that’s actually just recently finished enrolling, although we have no data yet to discuss, and I’m hoping that by the end of the year we will. So 21 hospitals across four countries, Australia, New Zealand, Singapore, and Canada, have contributed to this. And the concept is that if we can give Budesonide, a corticosteroid, combined with Surfactant and squirt it into the lungs, that will reduce death and BPD in this really fragile population.
Carol Vassar, podcast host/producer:
So this could really be a huge milestone in treating preterm babies should it come to pass that the two previous trials are replicated by PLUSS and what you’re doing right now.
Dr. Brett Manley, University of Melbourne:
Well, of course, we’re super hopeful that’s the case. I’m a little bit nervous, to be honest. I think it’s hard not to be cynical in our profession where over many decades, we’ve really struggled to reduce the incidents of BPD or make it go away. But having seen that Dr. Wei’s two trials and just how promising those results were, we’re very hopeful. Sorry. And I think the other thing about PLUSS is that because we’ve got more centers involved in four countries and we’ve also used a variety of respiratory care techniques and a variety of ways of giving the Surfactant and Budesonide, I think there’s going to be a lot of useful information in this trial when we finally got the results by the end of the year.
Carol Vassar, podcast host/producer:
When you’re enrolling, and I know you’ve just completed enrolling when you’re enrolling, how do you find the children and families? How do you convince them that this is something that could be helpful to children writ large?
Dr. Brett Manley, University of Melbourne:
Gosh. We could talk for informed consent for clinical trials, we could talk about that forever, but I think there’s some important things to talk about here. It’s really difficult, that’s the first thing. So these are families dealing with the imminent birth of an extremely preterm baby or even dealing with the recent birth of an extremely preterm baby. And if we use the PLUSS trials as an example, we approach families both before a birth when we thought that they were going to deliver an extremely preterm baby and very soon after the birth, sometimes in the first hours, although we did have the advantage of having up to a day or so to approach families for the trial, but many other clinical trials going on in neonatal medicine looking at interventions even before birth or in the delivery room or straight after birth, and therefore generally require informed consent from families that early in the infants lifetime.
It’s really difficult. Families are very stressed. As a result, most trials struggle to recruit; I guess a good recruitment rate or enrollment rate in many clinical trials would be around 50%. The problem with that, of course, is that that may or may not lead to a generalizable sample. We might be given the fact that we only recruit about half of eligible infants or families to some of our trials. That means half of eligible infants and families were not in the trial and may have been very different or may have had other reasons for not being in the trial. That means that when we try and translate our findings into the real world, we find that it could even be harmful or not beneficial to everyone that we thought. So one of the hot topics in our field is looking at how we might obtain informed consent with different methods we’ve been using and discussing the use of techniques like deferred or retrospective consent.
This is where families are approached after their baby is born and even after the intervention is given. So this is quite controversial in the sense that families are not asked before the study has occurred or the intervention has occurred; they’re approached afterwards and then have the right to either remain in the trial or not. And even some trials using something called a waiver of consent, where consent is never asked of families. That sounds really controversial and strange, but there are laws in most countries about how this can be safely done. And, of course, laws around what sorts of studies and interventions can be done with the concept of protecting the patient and the family and not doing anything that would be of high risk to the patient or family.
But back to your original point, we prospectively consented all the families in the PLUSS trial. So that’s 1,060 families that were, well, not quite so many because there were some twins in the trial, but 1,000-odd families that were consented in the hours before and after the birth of their extremely preterm baby. And you can imagine how stressful that is. And it never ceases to amaze me or any other perinatal researcher how willing families are to contribute to research in that incredibly stressful situation.
Carol Vassar, podcast host/producer:
You were talking about informed consent. I’m curious as to informed consent across countries, PLUSS has four countries involved. Are the informed consent requirements a bit different?
Dr. Brett Manley, University of Melbourne:
Across countries, they are generally nationally they’re similar or the same. I’m not exactly familiar with the differences between states, for example, in the US. But between Europe, the US, the UK, and Australia and New Zealand, for example. There are a lot of similarities but also some differences. And I think in terms of willingness to approach those sorts of alternative consent methods, there is a difference. I think in the US, people have been very concerned about the potential risk or the controversy generated from using waivers of consent or deferred consent, whereas in Australia and New Zealand, I guess it’s been used a little bit more, but still not without controversy. And in fact, I’m talking about this tomorrow at the conference, just how do we get this mixture right? Because you might say, how dare anyone experiment on my baby? And of course, that’s the natural reaction to a study that doesn’t ask you for informed consent.
But on the other hand, parents tell us this is an incredibly stressful time. Can you just get on with it and do a study that is safe and is going to contribute to the health of my baby and other babies and to society and ask me about it afterwards when I’m feeling a bit better about the whole situation? And in certain circumstances, there are trials that have no added risk to the patient or the family but still great health benefits where guidelines in various countries would say that that would be a reasonable approach to use a waiver of consent.
Carol Vassar, podcast host/producer:
Certainly benefits to the child at hand and benefits to children moving forward, given that this is research that can be applied for children who are yet to be even conceived.
Dr. Brett Manley, University of Melbourne:
Yeah, that’s right. And look, it’s such a controversial area, but the other thing to think about, I guess, is that just like other forms of medicine where there are emergencies happening, you can think of the emergency department, or you could think of paramedics, or you could think of receiving CPR and someone has a heart attack and drops to the floor. The only way to do research in those situations is to do it, and there is often not the opportunity to gain informed consent in those situations. And neonatology is a bit like that; we’re dealing with an incredibly acute field. We’re having babies born at 23 and 22 weeks gestation, who, in some places, have very high mortality rates. And I think that we, of course, have to get the balance right and be guided by parents and ethics experts, but I think it’s also not acceptable to not do research in these babies where we need to improve mortality and morbidity. And the only way to do that is to find out how.
Carol Vassar, podcast host/producer:
Let me ask you this. From the clinical side, is there a patient or patient family that has stuck with you through your career who maybe inspires you to keep going? Because there must be really tough days.
Dr. Brett Manley, University of Melbourne:
There are some really tough days, but like you say, there are the families and the babies that stay with you on both sides of the coin. So there are those babies that stay with you sometimes in quite a pathological way where you wonder whether you could have done better, or it’s been a difficult interaction with the family, or things have happened that shouldn’t happen or rarely happen that have just been so devastating. And on the other side of the coin, there are those families that are forever grateful to the hospital and for the medical care that they’ve received. And in some cases, those babies are the ones where you thought things were going to be bad, and they turned out good. It’s such a fascinating specialty we’re in for that very reason. The ethical issues and consent is just one; the life or death type scenarios being proven wrong and being proven right sometimes is just as hard. And the journey that you go through with families, which is one of the great parts of the job, but there’s probably not many other jobs like that where you’re there from the start, and you go through this journey that sometimes months or years of the ups and downs of the intensive care unit.
Carol Vassar, podcast host/producer:
Have you seen some of these children grow up and grow up healthy and go on perhaps maybe you’re too early in your career to see the children you treated have children of their own?
Dr. Brett Manley, University of Melbourne:
Probably just a little bit too early for that. No, we do see these children do well, and I think that’s a really important message. Maybe I painted a picture of doom and gloom without meaning to, but no, look, on average, most babies do well, even the tiniest babies. And I think we’ve perhaps been a little bit too pessimistic. And as more and more data comes out showing that some of these babies, most of these babies, grow up, yes, they might have some health difficulties, but if their families and they as individuals are happy and describe a good quality of life, whether or not they might have something that you or I or society would describe as a disability, then that’s very satisfying. And that’s exactly what we hear, these children, as they grow up, tell us that I have a good quality of life, thank you very much. And the families tell us that too. And in general, they’re just ecstatic that everything happened the way it did.
Carol Vassar, podcast host/producer:
That must be rewarding.
Dr. Brett Manley, University of Melbourne:
Very rewarding. And to play devil’s advocate, it does go the other way as well. But even families who have children or children themselves who have what we would describe as disabilities, they get on with it. And I think we as clinicians are starting to recognize that we can get these babies through and that they can grow up and live long and happy lives. Of course, there has to be limits on neonatal practice. I briefly mentioned earlier we’re looking after babies born as early as 22 weeks gestation now, which is physiologically about as early as you can be born and still get oxygen into your lungs and into your bloodstream. But this is happening around the world, and there’s really a new wave coming through now of tinier and tiny babies that we’re learning about.
Carol Vassar, podcast host/producer:
How did you end up in medicine? How did you end up in neonatology? There’s got to be a story there.
Dr. Brett Manley, University of Melbourne:
Yeah. I’m the first doctor in my family, so it’s not something that I’ve been born into. I was always attracted to the idea of medicine. I found it to be probably an exciting job to do and an interesting job to do. And then, as I went through my training, I was very much drawn to pediatrics. I just loved looking after families and the kids and seeing them get well. But as years went by, I found myself really umming and ahhing between surgery or procedural type specialties and pediatrics, and then I did neonatology, and I found out I can combine the two in a specialty that has a lot of excitement, is never boring. There’s resuscitations going on every day. You still get the interactions with families that I was looking for, and so that’s how I ended up settling down in the NICU.
Carol Vassar, podcast host/producer:
You don’t have a family background in medicine. What attracted you to science? What attracted you to medicine? Was it the people, was it the science, was it the combination thereof?
Dr. Brett Manley, University of Melbourne:
Yeah, definitely. I’m definitely a people person. Love chatting, love communicating, love chatting fairly extroverted. Also a scientific thinker more than a sort of humanities type person. So I enjoyed that side of it. I think I’m someone who needs constant interest and excitement in my life, and I don’t think I would ever be the person that was behind a desk all day. Not that there’s anything wrong with that, but just not for me. So that’s how I was drawn to medicine in the first place. And then, of course, there’s no one doctor who’s the same as another. There’s so many different things you can end up doing in medicine, but I’ve really found a combination of acute critical care medicine with neonatology combined with a role that I have in research, which allows me to really sort of try and innovate and critically think as well.
Carol Vassar, podcast host/producer:
You are very active on Twitter and, in fact, brought together a group under the #neotwitter, that is active throughout the world in terms of posting their research, supporting one another in the work of neonatology. Talk about that group, your get-together recently, how it happened that you got together here.
Dr. Brett Manley, University of Melbourne:
Yeah. I never thought I was going to be a Twitter guy, just like I’m sure a lot of people out there who aren’t on Twitter or other social media apps, they think that as well. They think, why would I go on there and I don’t want to hear about my colleagues cats or what they did on the weekend. Joining Twitter and becoming active in Twitter and it fluctuates how active I am, but doing that is one of the best things I’ve done in my research career. And I guess there are two things I think about mainly when I say that. Number one definitely is the networking that opens up and the ability to meet new people and make new friends and make new collaborations. And us getting together as a group using a hashtag yesterday was just one such great example. We had people from all over the world coming together, putting faces to names or putting faces to handles as the case may be, and meeting each other in real life. And it was just absolutely a wonderful event.
I think the other thing is that perhaps clinicians and researchers don’t understand just how powerful social media is in keeping your own education up to date and staying up to date with the literature. Of all the ways I’ve ever tried to stay up to date with research in my field, Twitter is, by far, in a way, the most powerful. Today is an example, you’ve got people who are at the conference here in Washington are tweeting about the results of new studies when they’re allowed to. They’re being respectful of author’s wishes, of course. But when they’re allowed to, they’re tweeting about the methods and results of studies, and they’re disseminating those results very quickly and getting immediate feedback, and that’s just amazing. I’m not sure there’s any other way that you can do that. Another example was a few weeks back when we had a clinical question looking at designing a new clinical trial and put out a quick survey on Twitter and had 400 results from around the world in a matter of days. And when you actually try and do a survey in another technique or using online tools, it’s rare to get such a good response in our field. So it’s very powerful, but it’s also been personally very, very satisfying.
Carol Vassar, podcast host/producer:
Anything else you’d like to share from the research perspective, from the clinical perspective, from your experience here at PAS?
Dr. Brett Manley, University of Melbourne:
I just want to circle around back to some of the issues with consent, but I think really bigger than consent is about educating the public about just how important clinical research is. I think there’s the feeling out there in our specialty of perinatal neonatal medicine that you need to be super careful about doing any research in little babies, and I understand that because that’s a very emotive sort of thought, but it’s just absolutely critical to improve outcomes for our patients. Other fields have managed to integrate clinical research into their day-to-day running, oncology is a really good example. You’re diagnosed with cancer, and you need treatment, almost without fault, you’ll be randomized in a clinical trial because they’re constantly trying to improve their methods of treating what is a condition with high mortality and morbidity.
And I think of our field a little bit like that, and we need to think of ways that we can educate the public to the point where there’d almost be an expectation that if you were having a high-risk pregnancy or having a tiny sick baby, that we would be trying to find ways to improve care, not only of you were baby but of all the babies that come after. So I think I’ll leave you with that message.
Carol Vassar, podcast host/producer:
Dr. Brett Manley, thank you so very much for being with us today.
Dr. Brett Manley, University of Melbourne:
Thanks, Carol.
MUSIC: Well Beyond Medicine!
Carol Vassar, podcast host/producer:
I’m Carol Vassar, thanks for listening to our conversation on the cutting-edge research being done by our guest, Dr. Brett Manley, on new clinical interventions for premature babies. I have a nephew who has benefited from the interventions developed as a result of research done by people like Dr. Brett Manley. Born at just two and a half pounds, young Max is now a happy, healthy, soon-to-be 7-year-old. Is there a child in your life who has benefited similarly? Continue the conversation with us by leaving your voicemail at nemourswellbeyond.org. That’s nemourswellbeyond.org. Maybe you’ll hear your voice on an upcoming episode of the Well Beyond Medicine Podcast.
While you’re there, check out our other episodes, subscribe to the podcast, and leave a review. Thanks to Che Parker, Cheryl Munn, and Susan Masucci for this week’s production assistance. Join us next week as we talk with Dr. Beena Kamath-Rayne, vice President of Global Health for the American Academy of Pediatrics, about all things children’s health on a global scale. Until then, remember we can change children’s health for good, well beyond medicine.
MUSIC: Well Beyond Medicine